Background: Rabbit anti-thymocyte globulin (rATG) is used in allogeneic hematopoietic cell transplantation (alloHCT) to prevent graft versus host-disease (GVHD) and graft rejection. In the T-cell replete setting, post-HCT rATG exposure is variable with high exposures resulting in delayed CD4+immune reconstitution (CD4+IR) and higher mortality. The goal of this study was to estimate the rATG exposure in pediatric and adult recipients of ex vivo T-cell depleted CD34+ selected alloHCT and correlate it with outcomes to determine the optimal exposure.
Methods: We performed a retrospective analysis of patients who underwent their first myeloablative-conditioned ex vivo CD34+ selected alloHCT between 2008 and 2018. Post-HCT rATG exposure was estimated as area under the curve (AUC) (mg*d/L) using a validated population pharmacokinetic (PK) model (Admiraal et al.,Lancet Hematology 2017). Outcomes of interest were CD4+IR, defined as CD4+>50/uL at two consecutive measures within 100 days of HCT, non-relapse mortality (NRM), overall survival (OS), GVHD, and relapse. We evaluated the association between post-HCT rATG exposures and CD4+IR using a smoothed effect to define the optimal post-HCT rATG exposure. We subsequently analyzed outcomes in 3 post-HCT rATG exposure groups, <30 mg*d/L, 30-55 mg*d/L, and >=55 mg*d/L. Cox proportional hazard models and multi-state competing risk models were used for analyses.
Results: Of 554 patients included, 239 (43%) were female, median age at HCT was 49 (range 0.2 to 73) years and 425 (76.7%) received matched donor transplant, while 129 (23.3%) patients received mismatched donor HCT. In this cohort of patients, 515 (93%) underwent alloHCT for hematologic malignancies - leukemia: 356 (64.3%), myelodysplastic syndrome: 122 (22%), and other hematological malignancies: 37 (6.7%) while 39 (7%) underwent alloHCT for non-malignant indications. Total Body Irradiation based conditioning regimen was administered in 177 (32%) patients. Among all 554 patients, 540 (97%) attained engraftment. Median post-HCT rATG exposure was 47mg*d/L (range 0 - 101 mg*d/L).
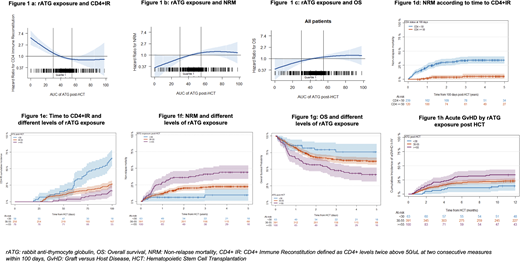
A decreasing post-HCT AUC (optimum <30 mg*d/L) was associated with higher probability of CD4+IR (p< 0.0001, Figure 1a); lower NRM (p=0.03, Figure 1b) and improved OS (p=0.05, Figure 1c). Patients who attained CD4+IR earlier had lower rates of NRM (p<0.0001, Figure 1d).
On further assessing rATG exposure post allo-HCT by three cut -off levels (<30mg*d/L, 30-55 mg*d/L and >=55 mg*d/L), time to CD4+IR varied depending on the ATG exposure (Figure 1e). In multivariable cox models, post-HCT rATG exposure >=55 mg*d/L was associated with an increased risk of NRM as compared to the lower exposure of <30 mg*d/L (HR: 4.11, CI: 1.52, 11.2, Figure 1f), and inferior OS (HR: 2.03, CI: 1.03,4.00, Figure 1g). In addition, post-HCT rATG exposure >=55 mg*d/L was associated with a higher risk of acute GVHD (HR: 2.28, CI: 1.01, 5.16, Figure 1h). In patients with hematologic malignancies, post-HCT rATG exposure was not associated with relapse (HR: 0.73, CI: 0.31,1.7). In the entire cohort of 554 patients, 9 (1.6%) patients had graft rejection: 1 primary rejection in the post-HCT ATG exposure <30mg*d/L group, 6 secondary rejections in the post-HCT ATG exposure of 30-55 mg*d/L group and 2 secondary rejections in the post-HCT ATG exposure >=55mg*d/L group. Among all patients, 204 (37%) died secondary to reasons such as relapse of disease: 73 (36%), infection: 51 (25%), GVHD: 40 (20%), toxicity/organ failure: 29 (14%) and other causes: 11 (5%).
Conclusions: In a large cohort of patients who underwent ex vivo CD34+ selected alloHCT, higher post-HCT rATG exposure led to higher NRM, a paradoxical increase in GVHD, and lower OS driven by poorer CD4+ IR. The increased rates of GVHD with higher post-HCT exposure may be related to increased infections in these cohorts, though this needs to be explored further. Individualizing rATG dosing by PK targeting to a low post-HCT rATG exposure may improve outcomes. We intend to validate these results in a forthcoming prospective clinical trial.
Scordo:McKinsey & Company: Consultancy; Angiocrine Bioscience, Inc.: Consultancy, Research Funding; Omeros Corporation: Consultancy; Kite - A Gilead Company: Other: Ad-hoc advisory board. Curran:Celgene: Research Funding; Novartis: Consultancy, Research Funding; Mesoblast: Consultancy. Kernan:Amgen: Current equity holder in publicly-traded company; Merck: Current equity holder in publicly-traded company; Johnson and Johnson: Current equity holder in publicly-traded company; Pfizer: Current equity holder in publicly-traded company. O'Reilly:Atara Biotherapeutics: Consultancy, Patents & Royalties: EBV-specific T-cell bank, Research Funding. Prockop:Mesoblast, Inc,: Consultancy, Honoraria, Research Funding; Atara: Research Funding; Jasper: Research Funding. Scaradavou:Excellthera: Membership on an entity's Board of Directors or advisory committees. Shah:Amgen Inc.: Research Funding; Janssen: Research Funding. Giralt:OMEROS: Consultancy, Honoraria; NOVARTIS: Consultancy, Honoraria, Research Funding; KITE: Consultancy; MILTENYI: Consultancy, Research Funding; ACTINUUM: Consultancy, Research Funding; TAKEDA: Research Funding; CELGENE: Consultancy, Honoraria, Research Funding; JAZZ: Consultancy, Honoraria; AMGEN: Consultancy, Research Funding. Perales:Medigene: Membership on an entity's Board of Directors or advisory committees, Other; NexImmune: Membership on an entity's Board of Directors or advisory committees; Omeros: Honoraria, Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Other; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria; Celgene: Honoraria; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotec: Research Funding; Kite/Gilead: Honoraria, Research Funding; Incyte Corporation: Honoraria, Research Funding; MolMed: Membership on an entity's Board of Directors or advisory committees; Cidara Therapeutics: Other; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bellicum: Honoraria, Membership on an entity's Board of Directors or advisory committees. Boelens:Bluebird Bio: Consultancy; Advanced Clinical: Consultancy; Race Oncology: Consultancy; Bluerock: Consultancy; Omeros: Consultancy; Avrobio: Consultancy; Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal